Importance of the ESCUDDO-CVT study:
Recently, the World Health Organization (WHO) launched the “Global Strategy to Eliminate Cervical Cancer as a Public Health Problem.” With this, the WHO intends to ensure that all girls in the world are vaccinated against HPV. Also, that all women over the age of 30 be screened and treated; this in case of having precancerous lesions. This study is of the utmost importance since it is part of the complementary research aimed at accelerating the elimination of cervical-uterine cancer as a public health problem.
Importance of the ESCUDDO-CVT study:
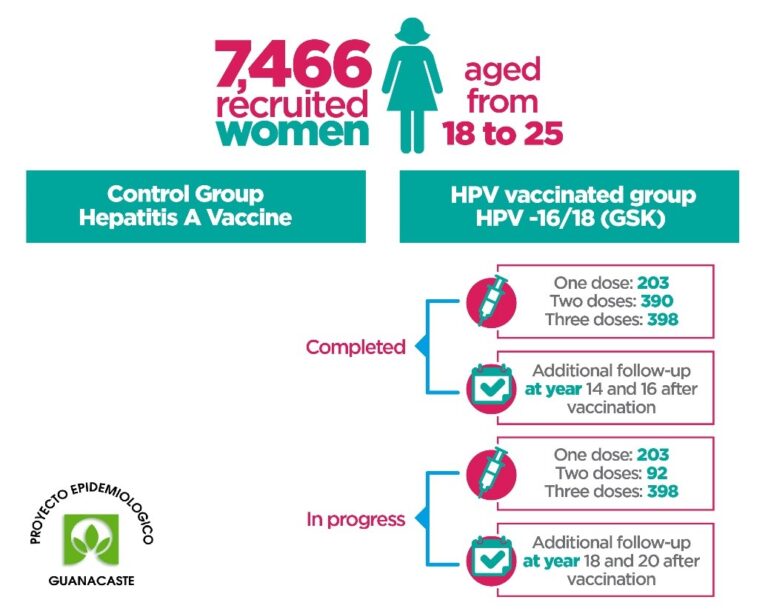
To monitor the positivity and stability of the antibodies against HPV 16/18 at a long-term (ie, 14, 16, 18, and 20 years after vaccination) among women vaccinated against HPV by number of doses (ie, 1 dose, 2 doses, and 3 doses).
Design of the ESCUDDO-CVT study:
Importance of the PRIMAVERA Study
A successful PRIMAVERA outcome, will provide earlier evidence than ESCUDDO, supporting single-dose schedules, accelerating HPV vaccine global adoption and the consequent reduction in cervical cancer worldwide.
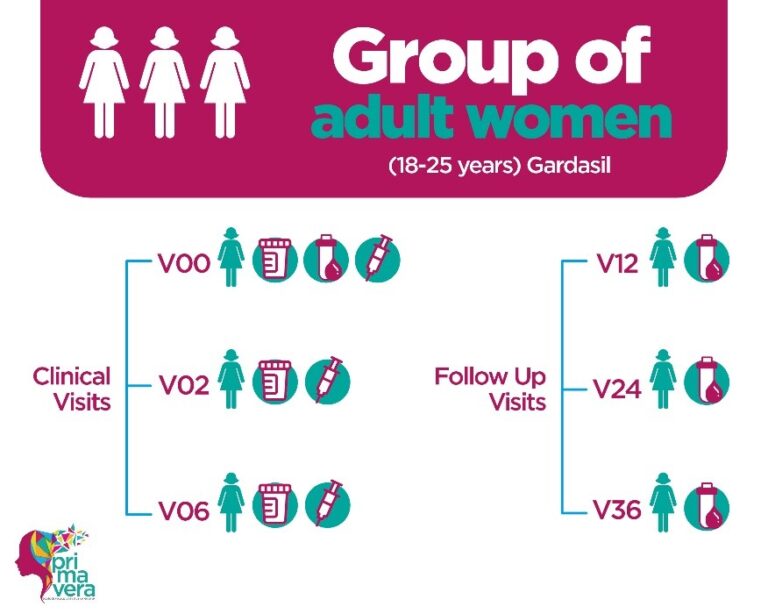
Objective of the study
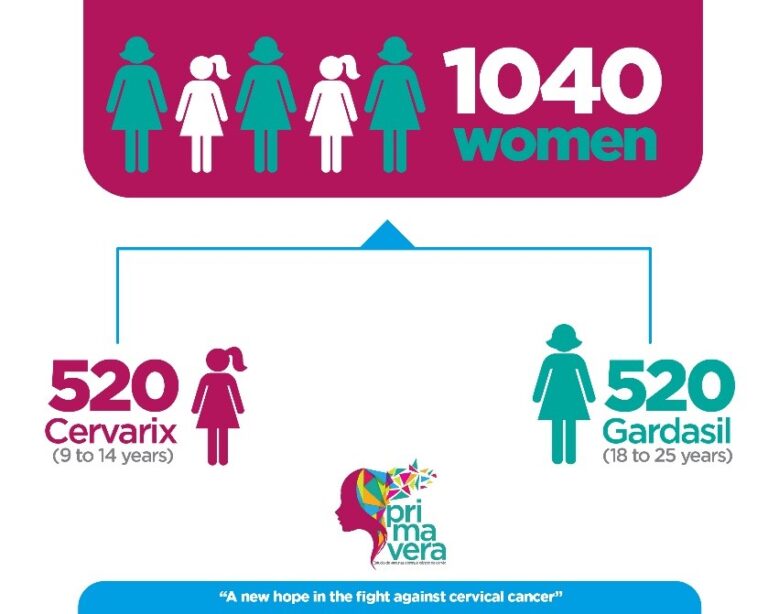
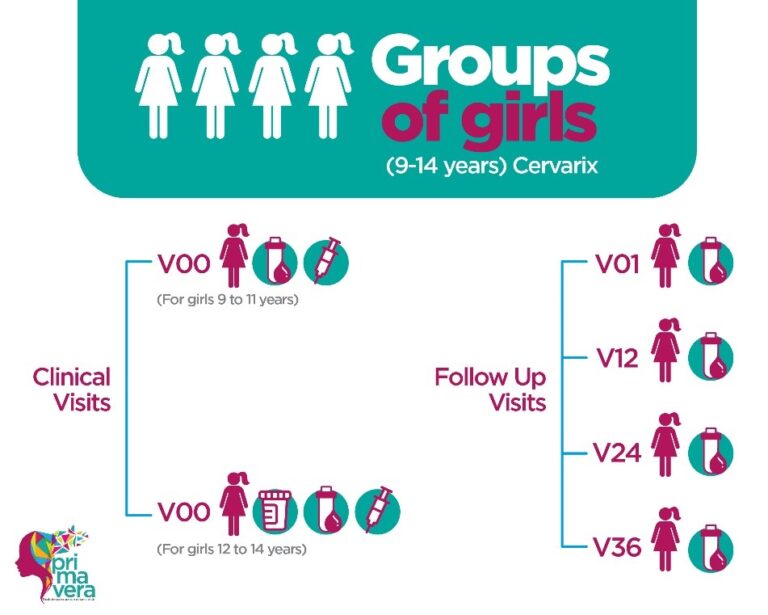
This study seeks to demonstrate that the immune response to 1 dose of Cervarix vaccine in girls 9-14 years of age is not inferior to the immune response to 3 doses of Gardasil vaccine in women 18-25 years of age, a combination dose/population with proven efficacy.
Design of the study
Randomization was not used in this trial because age indicates vaccine and number of doses. Therefore, there is no need to mask the vaccine.
Groups of women between the following ages were invited:
Related studies:
Importance of the LTFU Study
This research study answers the question: What is the global impact on cervical pre-cancer and cancer rates over a 10-year period of vaccinating young adult women against HPV?
The importance of this long-term evaluation comes from the first 4 years of follow-up of the CVT VACCINATION STUDY, which made it possible to evaluate the short-term impact of vaccination in relation to the presence of CIN3+ (severe cervical intraepithelial neoplasia, cancer in situ or invasive cervical cancer), so data on the protective effect of the vaccine beyond the first 4 years were required.
A formal evaluation of the impact of the vaccine over a period of 10 years would take the participating population of the CVT who agreed to continue their participation in the LTFU Study, up to the age of 28 to 35 years, thus covering the period of more high risk of CIN3+. In this way, the 10-year evaluation would provide a better estimate of the impact of vaccination “throughout life”, since most CIN3+ events occur at the age of 35 years in screened populations, therefore , if the evidence for the impact of vaccination at 10 years is high it would suggest that vaccination of young adult women would be justified and the estimates of the impact of vaccination at 10 years obtained in the LTFU would provide key information that could be used by economists in health to model the cost-effectiveness of vaccination programs.
Women who received less than 3 doses of HPV vaccine are of particular interest, as there is information from the CVT Study suggesting that 2 doses and up to 1 dose of vaccine may be sufficient to protect against HPV 16/18 infections.
Objectives of the LTFU Study
- Evaluate the 10-year global impact of vaccinating young adult women against HPV 16/18.
- Evaluate the determinants of the immune response to HPV and the vaccine and the markers of long-term protection.
- Evaluate the natural history of HPV infection and cervical disease in vaccinated and unvaccinated populations.
LTFU Study Design
The LTFU Study proposes a protocol for the 6-year extension of the follow-up period of some of the participants who were vaccinated against HPV 16/18 at the beginning of the CVT VACCINATION STUDY, who would have completed 4 years of participation, for a total of 10 years of active follow-up, with biannual visits, which will allow documenting relevant information on the risk factors and benefits of the prophylactic HPV vaccine.
This study included the participation of 6000 vaccinated and unvaccinated women, residents of the province of Guanacaste, Costa Rica and nearby areas.
Importance of the Natural History of Cervical Neoplasms Study
During the 90’s, the epidemiological evidence that cervical cancer and its precursor lesions behaved as sexually transmitted diseases (Brinton and Fraumeni, 1986), motivated multiple researchers for more than 20 years, to search for the agent biological transmissible and responsible for the etiology of this disease.
By 1993, various epidemiological and molecular biology studies had shown the existence of a relationship between HPV infections and the arrest of cervical neoplasms (Kaufman, 1989).
The natural history of precancerous lesions in the cervix relate the beginning of infection to HPV, which very often presents without clinical or histological manifestations.
To carry out the Natural History of Cervical Neoplasms Study, Costa Rica represented a special opportunity, for various reasons, among them the main ones were:
- It was a region with a high incidence of cervical cancer, and with areas of especially high incidence, such as the provinces of Guanacaste and Limón. (Sierra et al, 1988).
- The existence of a National Health System with an excellent infrastructure and the operation of Costa Rican population registries, as well as a high educational level, dimensions of the national territory, road network and telecommunications; that facilitate the performance of epidemiological studies at the population level.
- The epidemiology of cervical cancer in Costa Rica has been extensively studied.
Objective of the Natural History of Cervical Neoplasms Study
The main objective of this research study was to analyze the natural history of cervical neoplasia in high-risk populations, with emphasis on the role of the human papillomavirus (HPV) and other possible cofactors.
Design Natural History of Cervical Neoplasms Study
The study consisted in the invitation to participate of 12,000 women chosen at random from the population of women over 18 years of age living in the Province of Guanacaste, considered at that time as a region with a high risk of cervical cancer.
The participants in this study formed two groups, that of women with the presence of precancerous or malignant lesions of the cervix and that of women with the absence of these lesions or without pathology, who constituted the follow-up cohort in which the determining factors were analyzed of the appearance of preneoplastic lesions.
The research consisted of two phases, in the first phase the invitation to participate and inclusion or recruitment was carried out, during the first 18 months, and during the second phase the follow-up was carried out, which began simultaneously and would continue for 5 years.
Importance of the
CVT Vaccination Study
Human papillomaviruses (HPV), produce a very common infection, both in men and women, which usually heals on its own, but if it does not heal, it can cause cancer after a long time. Therefore, if a woman has an HPV infection in her cervix, there is a chance that she will develop cervical cancer, and as with other diseases that are caused by viruses, a vaccine can be effective in preventing infection and cancer.
Researchers from the National Institutes of Health of the United States discovered the HPV vaccine based on virus-like particles (VLP), which preclinical studies reliably demonstrated the preventive efficacy of this vaccine in relation to cervical cancer. Therefore, based on these promising findings, the National Cancer Institute (NCI) and ACIB-FUNIN carried out this CVT VACCINATION STUDY in Guanacaste, an area with high rates of cervical cancer and where in 1993 the Natural History Study of HPV and Cervical Neoplasia were carried out also, which demonstrated the association of HPV infection with the development of precancerous lesions and cervical cancer.
Objective of the
CVT Vaccination Study
To demonstrate the efficacy of vaccine candidate compared to control in preventing histopathologically confirmed CIN 2+ associated with cervical HPV16 or HPV18 infection after dose 3 (from month 6 to month 48), in women HPV DNA-negative young adults (by PCR) at months 0 and 6 for the corresponding HPV type of HPV.
(CIN 2+ is defined as cervical intraepithelial neoplasia or preinvasive lesion that produces changes in the transformation zone of the cervix, which can be CIN2 or moderate dysplasia, CIN3 or severe dysplasia, cancer in situ or invasive cervical cancer).
mbios en la zona de transformación del cuello de útero o cérvix, que pueden ser NIC2 o displasia moderada, NIC3 o displasia grave, cáncer in situ o cáncer cervical invasor).
Design of the
CVT Vaccination Study
The CVT STUDY is that of a randomized, double-blind, controlled clinical trial, whose population of interest were women between 18 and 25 years of age, who, to be included in this research study, had to be considered healthy according to medical judgment by the clinical study staff and who were not pregnant at the time of vaccination and were willing to use an effective contraceptive method 30 days before vaccination until 60 days after the last vaccination.
Participants were vaccinated three times over a six-month period (at months 0, 1, and 6) with either the HPV vaccine (Cervarix) or the hepatitis A vaccine (Havrix). At each of these visits, blood samples for evaluation of antibody titers. As part of the active monitoring of participation, each woman made annual visits to the ACIB-FUNIN care centers for four years.
Related Resources
Publications
- Shing JZ, et al. Lesiones cervicales precancerosas causadas por tipos de VPH no prevenibles por vacunación después de la vacunación con la vacuna VPH bivalente con adyuvante AS04: un análisis del estudio de seguimiento a largo plazo del ensayo aleatorizado de vacunas contra el VPH de Costa Rica. Lanceta Oncol
- Porras C, Tsang SH, et al. Eficacia de la vacuna bivalente contra el VPH contra el precáncer asociado al VPH 16/18: resultados del seguimiento a largo plazo del ensayo de vacunas de Costa Rica. Lancet Oncol
- Kreimer AR, Sampson JS, et al. Evaluación de la durabilidad de una dosis única de la vacuna bivalente contra el VPH: el ensayo CVT. JNCI
- Tsang SH, Sampson JS, et al. Durabilidad de la protección cruzada por diferentes esquemas de la vacuna bivalente contra el VPH: el ensayo CVT. JNCI
- Herrero R, Wacholder S, Rodríguez AC, et al. Prevención de la infección persistente por el virus del papiloma humano mediante una vacuna contra el VPH16/18: un ensayo clínico aleatorizado basado en la comunidad en Guanacaste, Costa Rica. Descubrimiento del cáncer
- Herrero R, Hildesheim A, Rodríguez AC, et al. Justificación y diseño de un ensayo clínico aleatorizado doble ciego basado en la comunidad de una vacuna contra el VPH 16 y 18 en Guanacaste, Costa Rica . Vacuna 2008.
Pending publication of results of this scientific research.