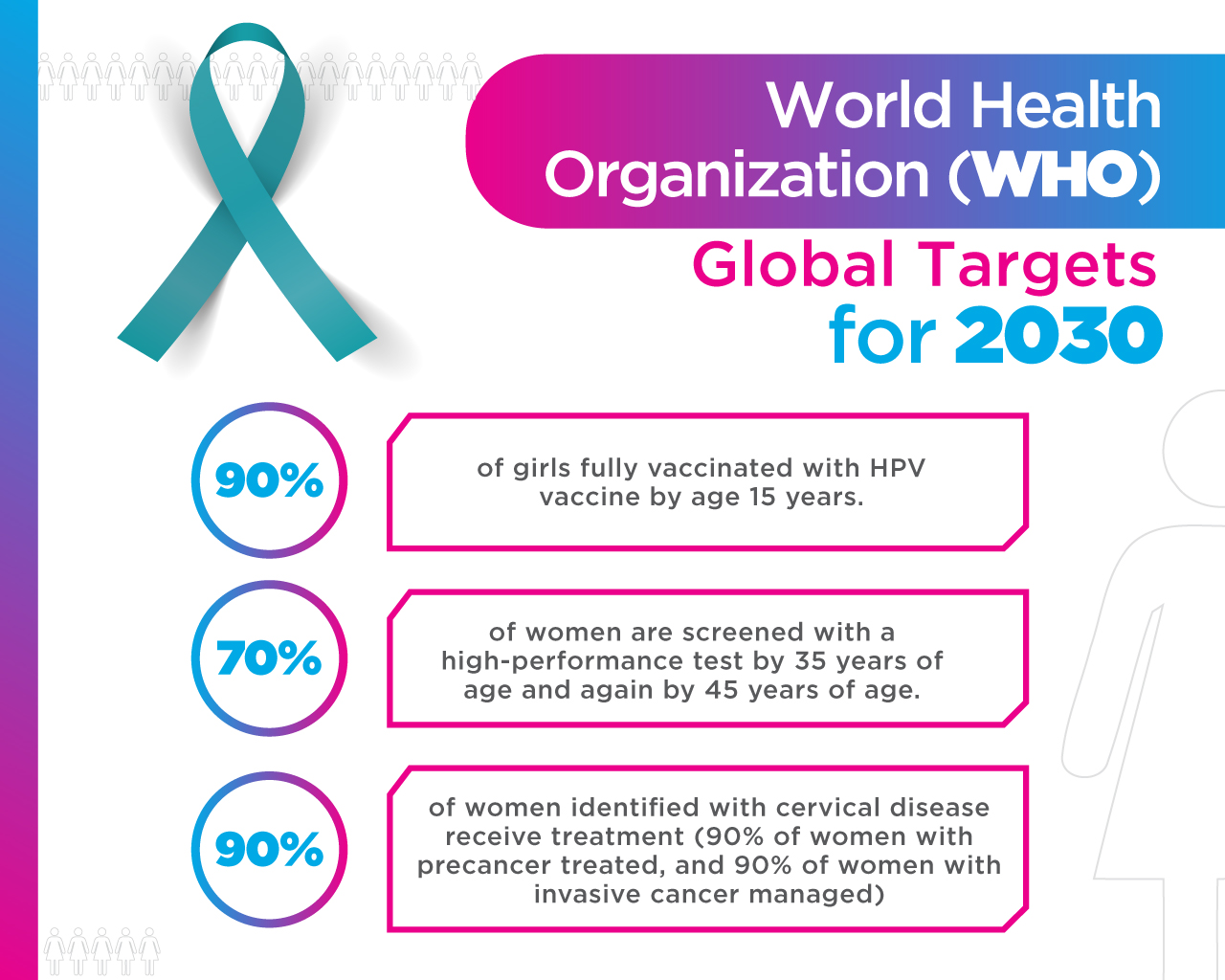
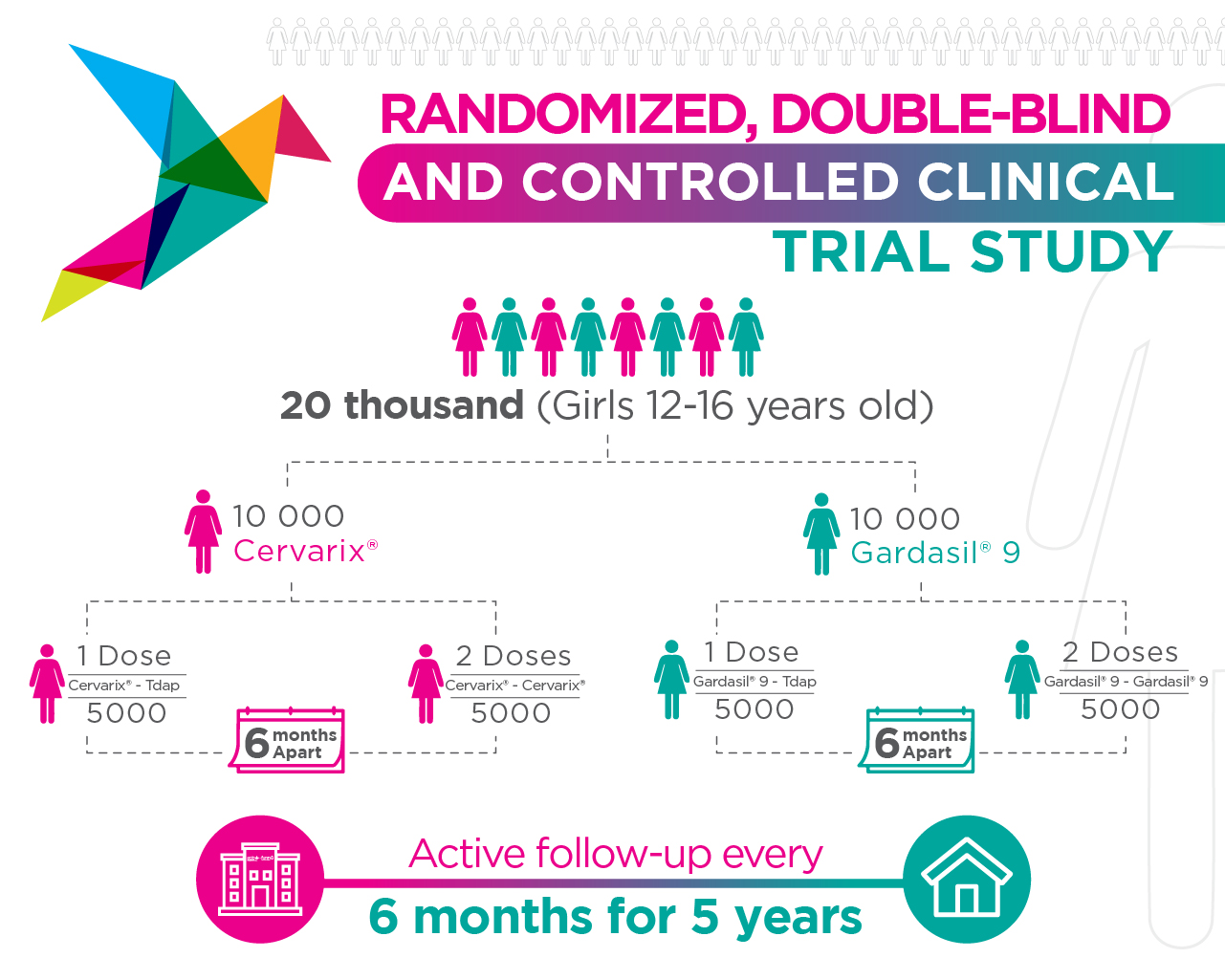
What is the ESCUDDO Project?
The ESCUDDO Project is a randomized clinical trial that began in November 2017 and included 20,330 female adolescents between 12 and 16 years of age from 202 districts of Costa Rica. Its objective is to evaluate whether a single dose of the approved HPV vaccines (Cervarix® and Gradasil-9®) is equally effective as two doses.
ESCUDDO means: Comparative Study of One and Two Doses of Vaccines Against Human Papillomavirus (HPV).
Principal Investigators of ESCUDDO Study:MSc. Carolina Porras Gutiérrez, ACIB-FUNIN and Aimee R. Kraimer. Ph.D, NCI.
Sponsor: National Cancer Institute of United States (NCI).
Institutions participating in the ESCUDDO Project: National Cancer Institute of United States (NCI); Agencia Costarricense de Investigaciones Biomédicas (ACIB-FUNIN); International Agency for Research on Cancer; and World Health Organization.
Approved by the Scientific Ethics Committee: CEC-HCB-E006-2024
Registration in ClinicalTrials.gov NCT03180034
Clinics and Doctors of the ESCUDDO Project