What is the
RESPIRA Study?
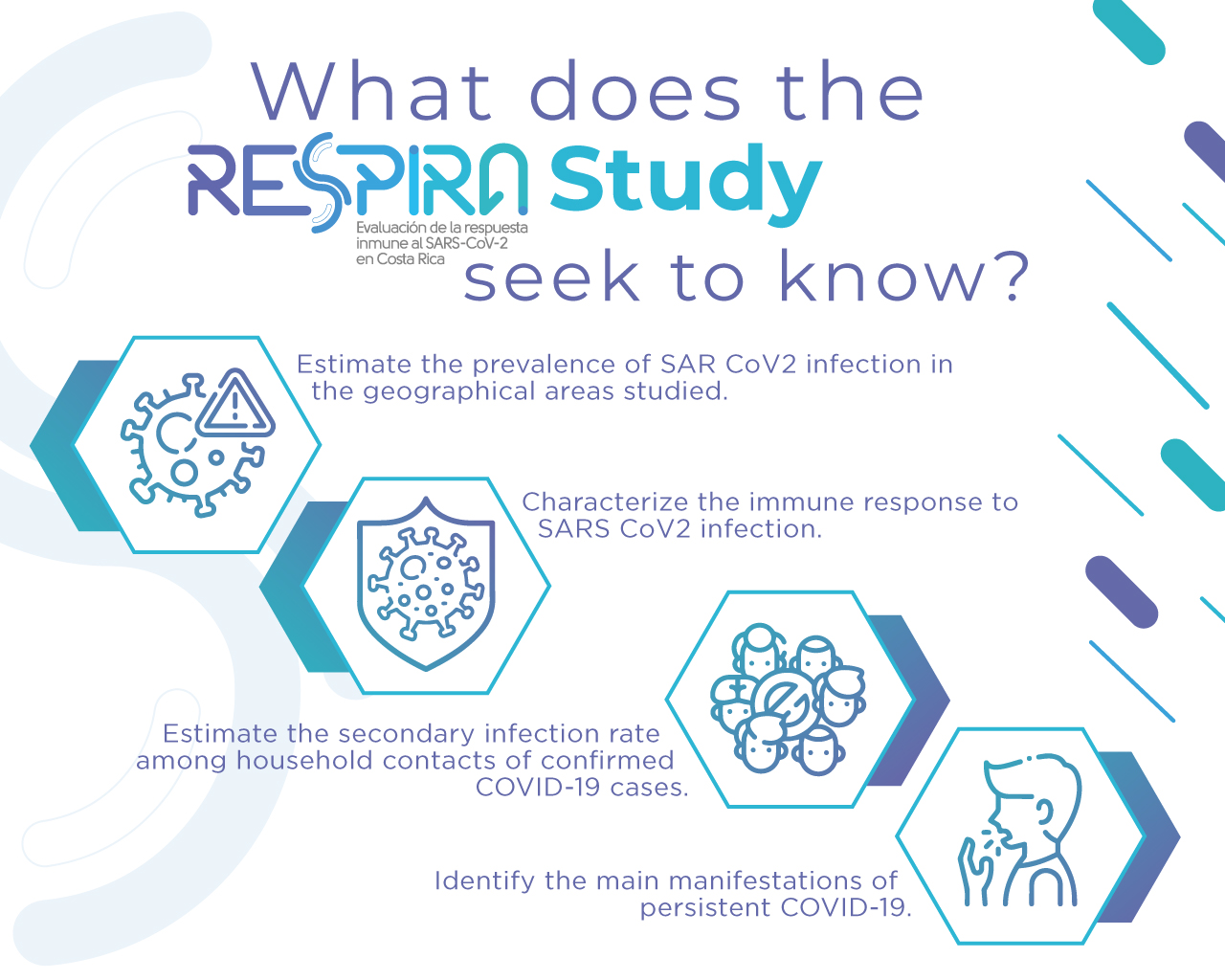
The RESPIRA Study is an observational investigation of cases and controls in Costa Rica. Its objective is to evaluate the immune response to the SARS-CoV-2 virus that causes the COVID-19 disease.
The RESPIRA Study began in November 2021 and involved the participation of 999 people (both children and adults) randomly chosen among those who were diagnosed with COVID-19 by PCR test in Costa Rica; this from March 2020 to November 2021.
Likewise, 1999 people were included in the study, who had not suffered from the disease, but who had the same sex, age and place of residence as the cases. Also, 719 contacts residing in the same household of 300 of the cases were included to evaluate secondary transmission.
An important detail of the RESPIRA Study is that the participants had to reside in selected districts of the Great Metropolitan Area (GAM), Guanacaste and Puntarenas.
RESPIRA means: Evaluation of the Immune Response to SARS-CoV-2 in Patients with COVID-19 in Costa Rica.
Principal Investigators: Dr. Amada Aparicio Llanos (CCSS) and Rolando Herrero Acosta, Ph.D, ACIB-FUNIN.
Sponsors: Costa Rican Social Security (CCSS); Research and Technological Innovation Fund of the CCSS; and Costa Rican Agency for Biomedical Research - INCIENSA Foundation (ACIB-FUNIN).
Institutions participating in the RESPIRA Study: Costa Rican Social Security (CCSS); Ministry of Health of Costa Rica; Costa Rican Agency for Biomedical Research (ACIB-FUNIN); US National Institutes of Health (NCI y NAIDS); German Cancer Institute; and University of Costa Rica (UCR).
Approved by the Scientific Ethics Committee: CEC-Central-CCSS R020-SABI-00261.
Registro en ClinicalTrials.gov NCT04537338
Summary of Achievements