What is the PRIMAVERA Study?
The objective of this research is to provide convincing and actionable evidence to regulators that a single dose of HPV vaccine gives an immune response sufficient to protect against specific HPV infections and subsequent malignancies. This study will provide earlier and complementary results to the study currently being conducted in Costa Rica called ESCUDDO, which is being conducted by the National Cancer Institute of EE.UU. (NCI) and the Costa Rican Agency for Biomedical Research (ACIB), with the participation of 24,000 girls and evaluates the efficacy of the vaccine for a 1-dose scheme against virological results and is estimated to be completed in 2024/5.
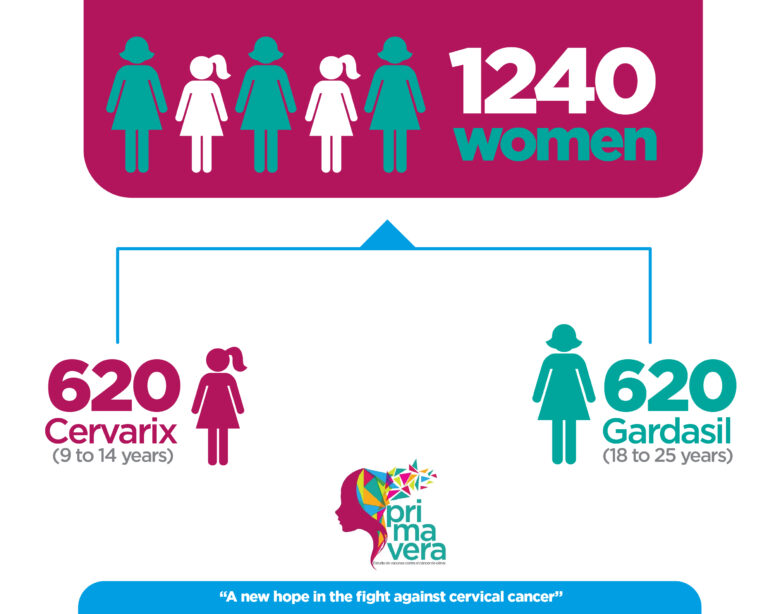
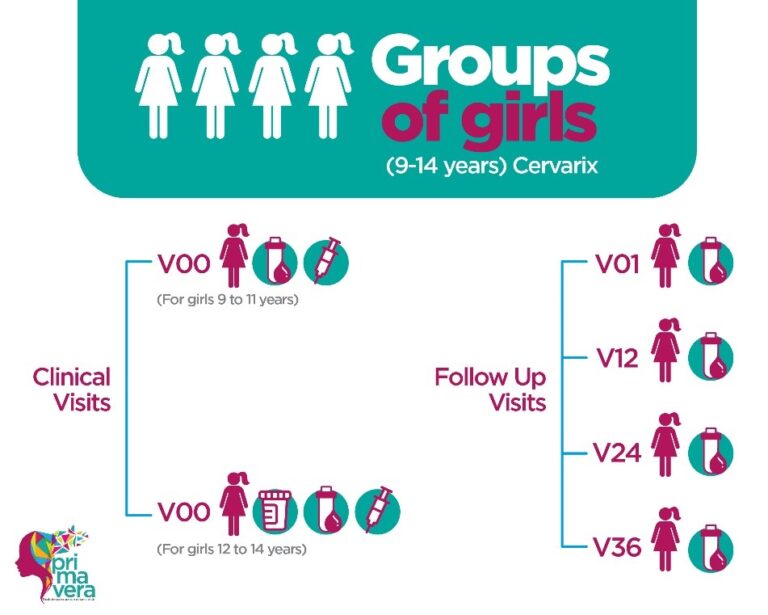
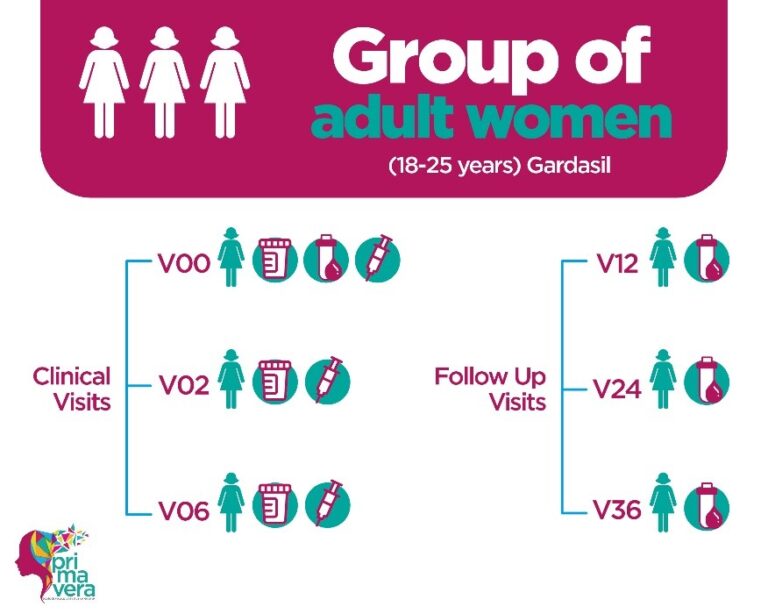
PRIMAVERA is a study that seeks to generate early evidence on the effectiveness of a single dose of the bivalent HPV vaccine in the prevention of cervical cancer. We recruited 520 young adult women who received 3 doses of the quadrivalent HPV vaccine, Gardasil, and 520 girls between 9 and 14 years of age who received one dose of the bivalent vaccine, Cervarix.
Subsequently, they will have annual visits for 3 years for blood collection, which will be used to measure the levels of circulating antibodies and the levels of antibodies that will be used to compare the immune response exerted by a single dose of the bivalent vaccine in the group of girls against the immune response exerted by 3 doses of the quadrivalent vaccine in adults.
PRIMAVERA is a member of the ESCUDDO family of studies (ESCUDDO, ESCUDDO-CVT and PRIMAVERA-ESCUDDO) carried out in Costa Rica by ACIB-FUNIN in exceptional collaboration with the NCI.
PRIMAVERA means: “Non-inferiority trial comparing the immunogenicity of 1 dose of bivalent HPV vaccine in girls with 3 doses of quadrivalent vaccine in women: The Immune Response Bridge Trial to improve access to vaccines and eradicate cancer (the PRIMAVERA - ESCUDDO study)”
Principal Investigators of PRIMAVERA Study: MSc. Bernal Cortés Ledezma, ACIB-FUNIN y Aimée R. Kreimer, Ph.D, NCI.
Sponsor: National Cancer Institute of United States (NCI)
Institutions participating in the PRIMAVERA Study: National Cancer Institute of United States (NCI), National Institutes of Health, Costa Rican Agency for Biomedical Research (ACIB-FUNIN), International Agency for Research on Cancer, World Health Organization.
Approved by the Scientific Ethic Committee: CEC-HCB-E008-2024