Importancia del estudio
asdasdas

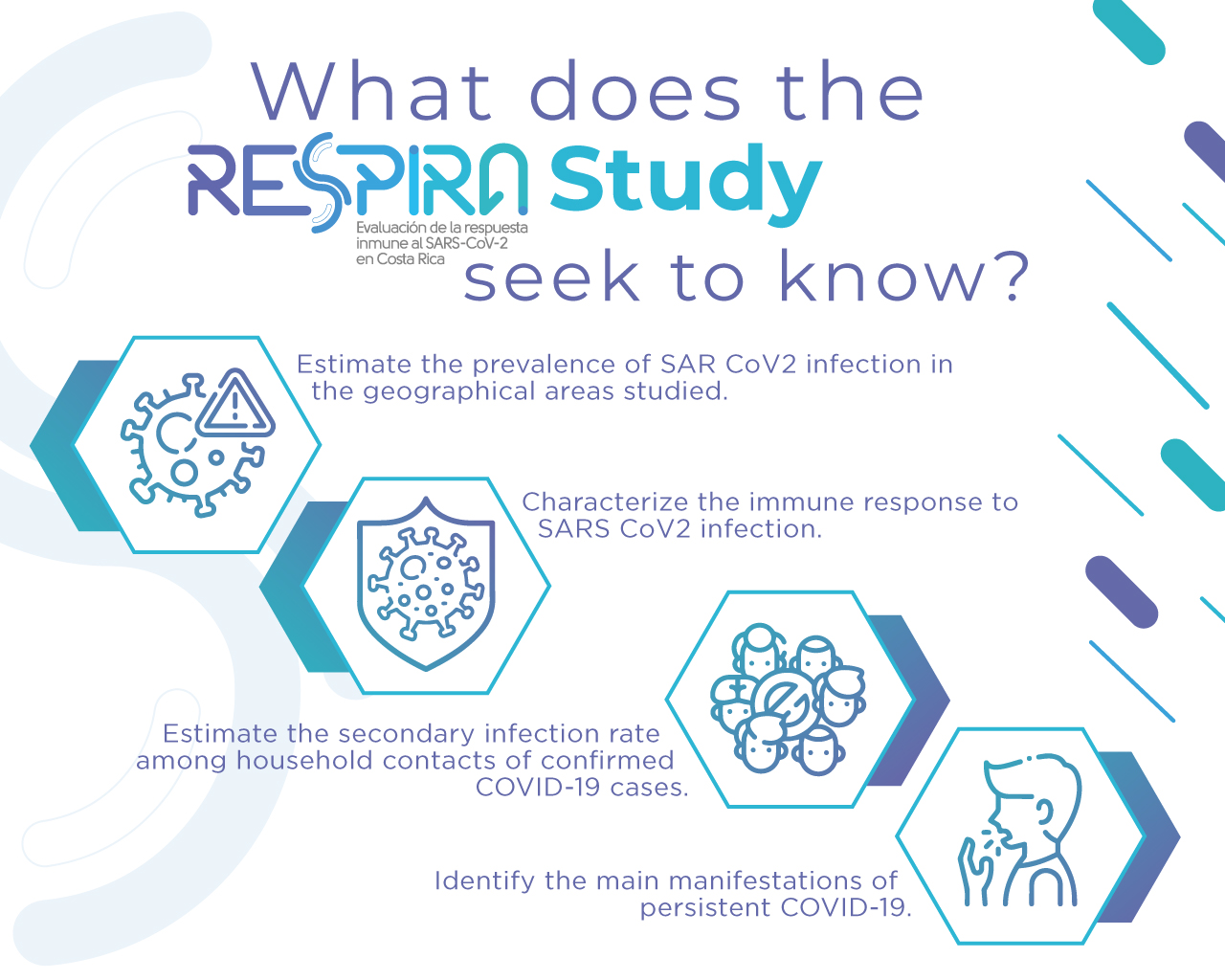
Importance of the RESPIRA Study
The RESPIRA Study seeks to provide knowledge to the national and global community about the characteristics of the immune response to this virus, which includes the levels and types of antibodies required to protect people against infection by the SARS-CoV-2 virus and its duration. In this regard, it should be remembered that the COVID-19 disease has affected and caused the death of millions of people around the world.
Objective of the RESPIRA Study
To determine the immune response to the SARS-CoV-2 virus in terms of antibody levels, differences in the response related to epidemiological and clinical characteristics, the duration of the response and the efficacy of protection, as well as its determinants, genetic characteristics and transmission secondary to household members.
The RESPIRA Study is an observational study of cases and controls
RESPIRA invited almost 3,000 people to participate, including children and adults, Costa Ricans and foreigners residing in Costa Rica, who will be followed up for two years. The objective is to constantly evaluate their levels of antibodies or defenses and determine if there is a new appearance of the virus. The groups of participants are mainly divided into cases and controls, with a third group of household surveys, which is explained below:


Importance of the PRISMA Study
Recently, the World Health Organization (WHO) launched the “Global Strategy to Eliminate Cervical Cancer as a Public Health Problem”. With this, the WHO wants to ensure that all girls in the world are vaccinated against HPV. Also, that all women over the age of 30 be screened and treated; this in case of having precancerous lesions.
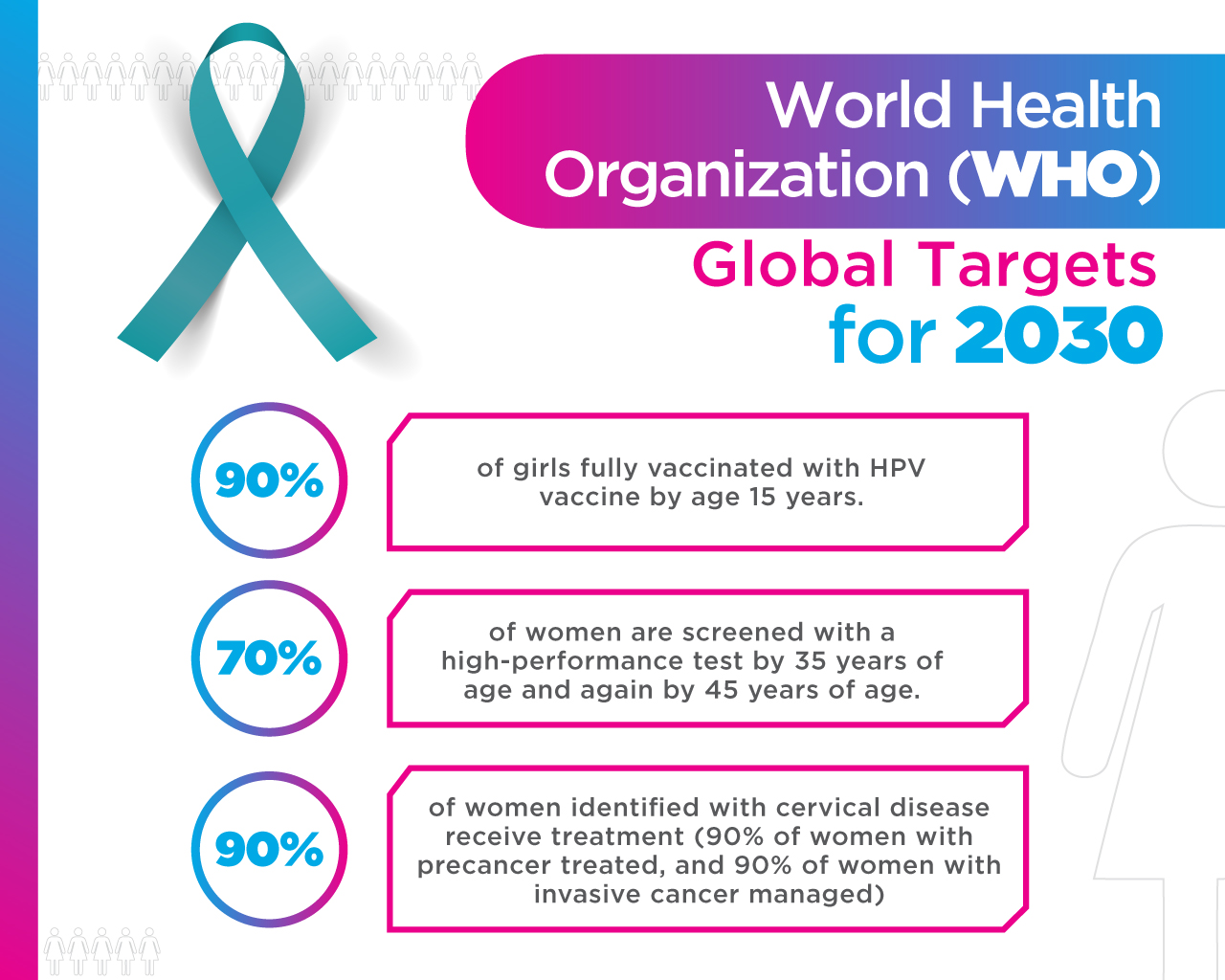
In relation to this matter, vaccination against HPV in adult women is not cost-beneficial for the countries. That is, the cost is high and the benefit is lower. This is mainly due to two reasons:
1. The current recommendation in young adult women is to apply three doses of the vaccine.
2. The longer a woman has been sexually active, the less benefit the vaccine offers in protecting against HPV 16 and 18. This is because HPV infection is very common and is acquired in the years after the beginning of sexual life.
So, with the PRISMA Study it is expected to know if a single dose of the HPV vaccine can prevent young adult women from being infected with HPV. In this sense, it will be possible to evaluate the benefit of vaccination up to the age of 30, with the goal of providing data so that the entities that emit recommendations can consider changes in their policies. Likewise, vaccination of young women could reduce the number of years it would take to eliminate cervical cancer as a public health problem in the world.
Objective of the PRISMA Study
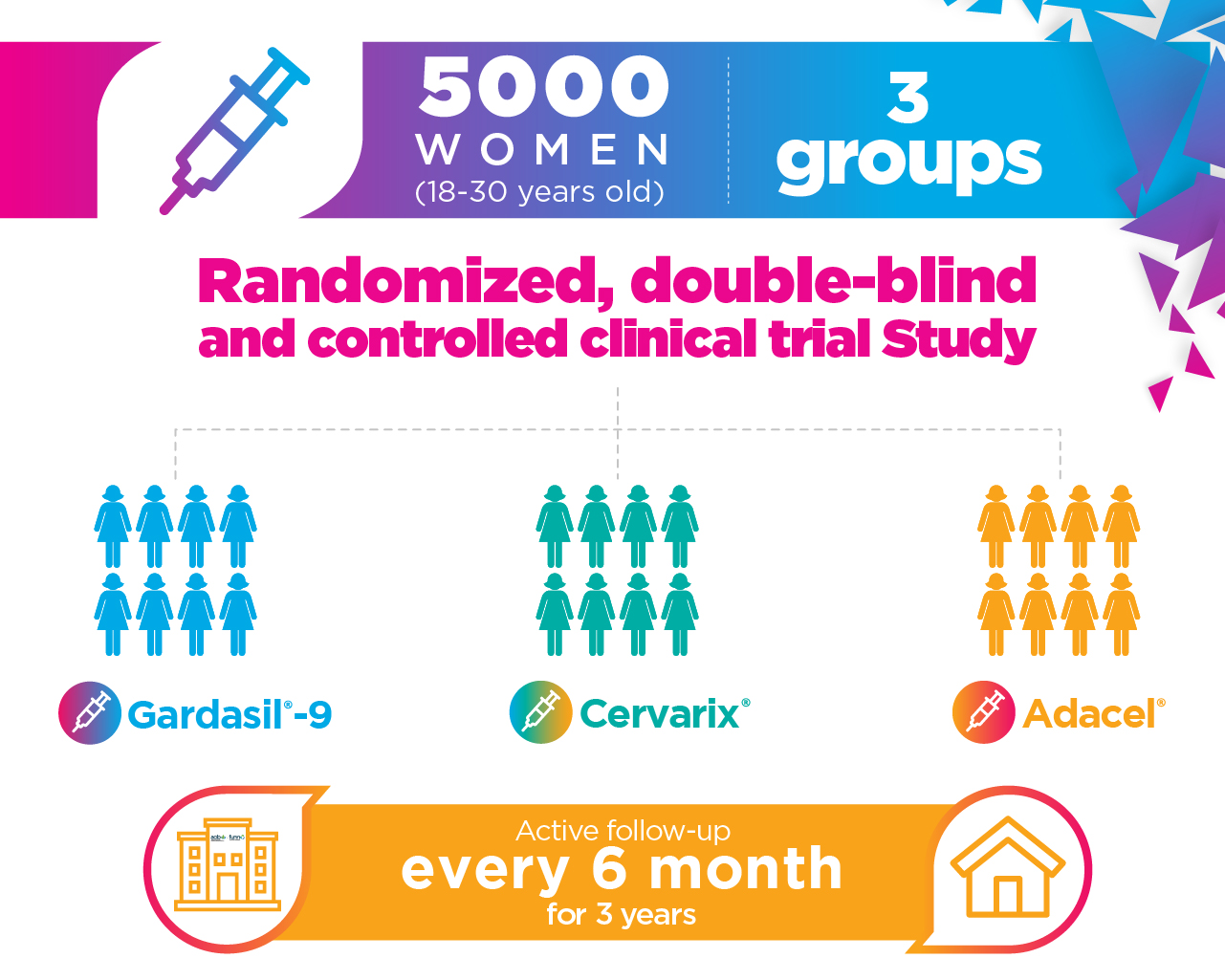
Evaluate a single dose of the vaccines against HPV (Cervarix® and Gradasil-9®) compared with no vaccination to protect against incident cervicovaginal HPV type 16/18 infections persisting for six months or more, in women aged 18 to 30 years, who are negative for HPV types 16/18, before and at the time of vaccination.
Design of PRISMA Study
The participants will be randomly assigned to one of the three study groups.
Importance of the ESCUDDO Project
Recently, the World Health Organization (WHO) launched the “Global Strategy to Eliminate Cervical Cancer as a Public Health Problem”. With this, the WHO aims to ensure that all girls in the world are vaccinated against HPV. Also, that all women over the age of 30 be screened and treated; this in case of having precancerous lesions.

Vaccination of adolescent females against HPV is the most effective long-term intervention to reduce the risk of cervical cancer. However, the majority of adolescent girls in the countries with the highest cervical cancer death rates are not being vaccinated. The foregoing, due to cost and logistics considerations, related to implementing multidose programs in adolescents, which prevents progress in reducing this preventable cancer.
With this, the ESCUDDO Project seeks to provide the necessary scientific evidence to modify global policies towards single-dose HPV vaccination programs.
¡One single dose could be enough!
Objective of the ESCUDDO Project
1. To evaluate the non-inferiority of one dose versus two doses of each of the two HPV vaccines.
2. To estimate the efficacy of one dose of the vaccine versus no vaccination.
Design of ESCUDDO Project
This section involves two main components:
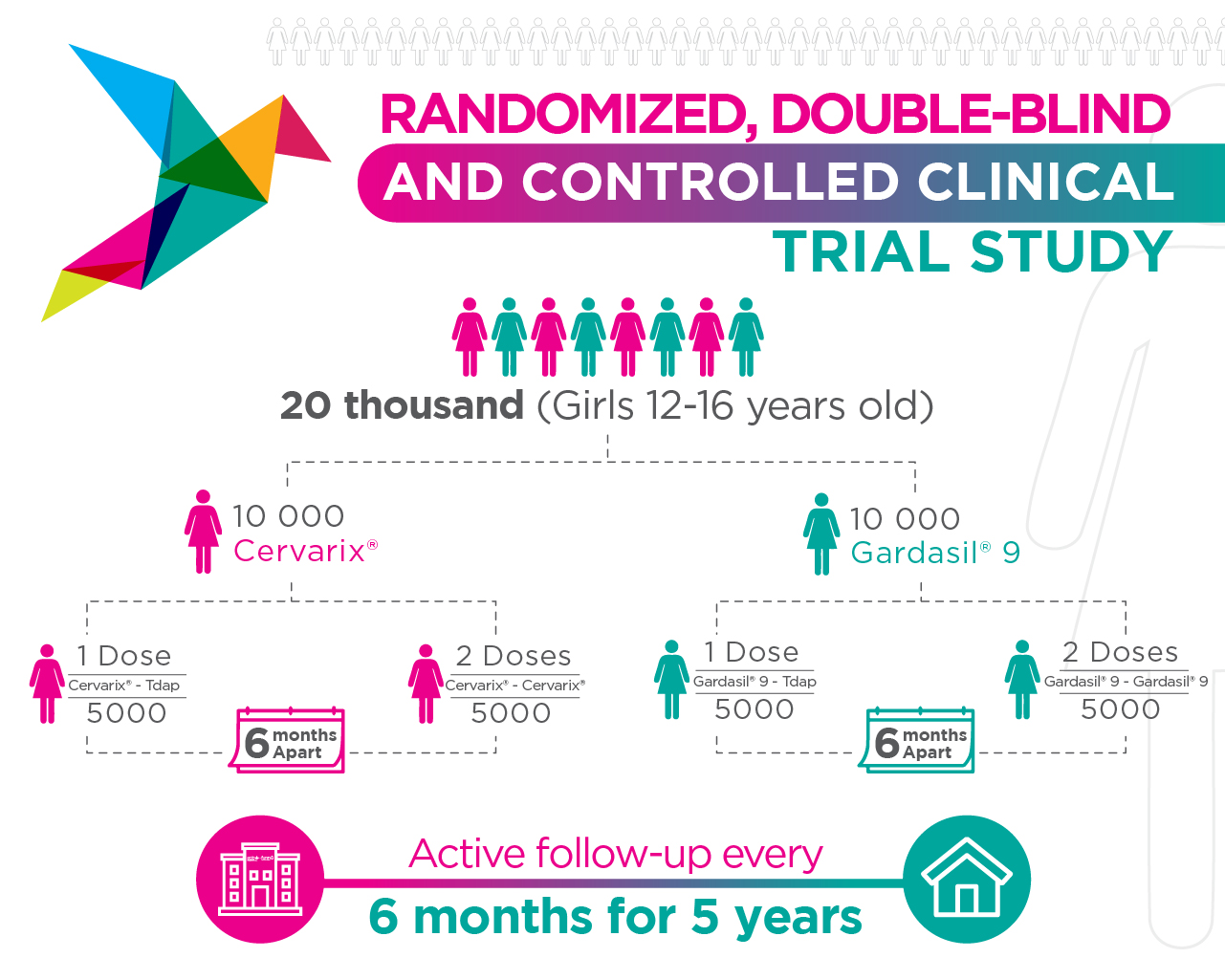
1. Clinical trial.
- Participation of adolescent women from 12 to 16 years of age.
- These women attend semi-annual visits for five years.
- Will compare the protection conferred by vaccination with one dose and two doses of the HPV vaccine.
2. Epidemiological surveys.
- Participation of women from 16 to 21 years of age.
- These women attend two visits six months apart.
- Will determine the presence of HPV among unvaccinated women.
- An epidemiological survey was included at the beginning of the ESCUDDO Project and another will be included at the end.