What is the ESCUDDO-CVT study?
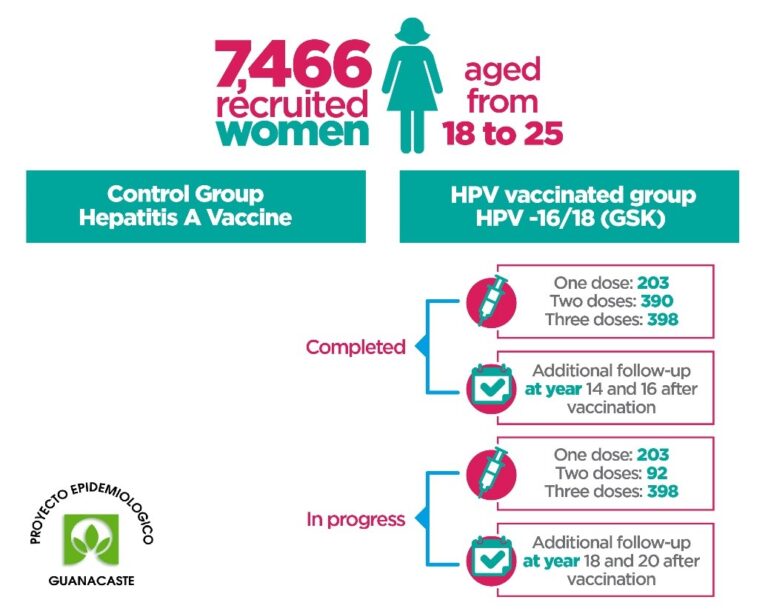
The ESCUDDO-CVT study is an observational long-term immunological follow-up study of women who received one, two, and three doses of the bivalent vaccine against Human Papilloma Virus (HPV) in the CVT (HPV-16/18 Vaccination Trial Costa Rica – former Guanacaste Epidemiological Project).
ESCUDDO-CVT means: “Long-term immunological follow-up of women who received one, two and three doses of the bivalent HPV vaccine in the CVT (Costa Rica HPV-16/18 vaccination trial): Generating durability data : the “ESCUDDO-CVT” study
Principal Investigators of ESCUDDO-CVT Study: Dr. Byron Romero Robles, ACIB-FUNIN and Aimée R. Kreimer, Ph.D, NCI.
Sponsor: National Cancer Institute of United States (NCI), National Institutes of Health (NIH).
Institutions participating in the ESCUDDO-CVT study:National Cancer Institute of United States (NCI), National Institutes of Health (NIH) and Costa Rican Agency for Biomedical Research (ACIB-FUNIN).
Approved by the Scientific Ethics Committee: CEC-HCB-E007-2024